Our lab works in the area of virus immunology. The goal is to understand the development of long-term sustained humoral immunity. The research is focused on understanding the dynamics and biology of CD4+ T cells, mainly “follicular T helper (Tfh) cells”, in the settings of long-term immunological memory. Our research program on virus infection vs controlled human vaccination provides a unique opportunity to identify the positive attributes of sustained immunity. We are integrating deep immunophenotyping, T-cell functional assays, immunogenomics, and metabolomics approaches to investigate the biology of Tfh cells in longitudinal cohorts of individuals with virus infection and vaccination. For mechanistic investigations, we use the Tfh cell ex-vivo setup and the antigen-specific/transgenic mouse models. While the lab’s research has focused on exciting questions, we have made contributions outside of the core research efforts of the lab. We have established a “Human Immune Monitoring and T-cell Immunoassay” platform, with applications for the advanced immunological human trials of vaccines. The global aim of our research is to provide foundational knowledge for the rational design of human vaccines.
About
Members
Dr. Nimesh Gupta
Chief of Laboratory
Bhushan S Nikam
PhD Scholar
Someshwar Nath Jha
PhD Scholar
Suvechchha
PhD Scholar
Bhavya Ahuja
PhD Scholar
Shayon
iBRIC PhD Scholar
Vivekananda
PhD Scholar
Samiya
PhD Scholar
Eileen Kaur
Intern
Prakhar Varshney
Trainee
Ananya Banerjee
Trainee
Lab Feed
Our recent dengue research featured in the Editor’s Choice section of Science Immunology
New Immune Pathway in Dengue : A Breakthrough for Next-Gen Vaccines
Research Programs
Tfh cells in long-term sustained immunity in vaccination and infection.
Japanese encephalitis is a major socio-economic concern for our country. Though few vaccines exist for JE, they have limitations in developing effective long-term protection. By studying the immune response to the historical live attenuated SA14142 JE vaccine, our lab has helped in establishing the mechanism of protective immunity conferred by the vaccine (Eur J Immunol, 2020). We are investigating the longitudinal cohort of licensed human SA14-14-2 live attenuated Japanese encephalitis (JE) vaccine and JE patients in the northeast region of India. The aim is to define the characteristics and clonotypes diversity of human Tfh cells in sustained immunity established in response to vaccination and natural infection. We have found that the quality and durability of humoral immunity is more enriched in JEV infection than in vaccination.
Research Strategy for exploring the biology of Tfh cells in infection vs vaccination.
There are interesting leads that indicate the key role of Tfh cells in the magnitude and durability of humoral immunity against JEV. We are in the process of probing the unique traits of JEV-specific Tfh cells in functional T cell immunoassays and by employing single-cell RNASeq and the metabolomics approaches. In a parallel study, we are also trying to understand the immunological reasons underlying non- responders (~18%) to live attenuated JE vaccine. We are applying integrated analyses approach with transcriptomics, proteomics and metabolomics for an in-depth understanding of the underlying immunological determinants. We have found a metabolic association with no response to vaccination. Further in-depth investigations are in progress in the JEV challenge and immunization animal models.
Biology of Tfh cells in humoral immunity establishment to dengue virus
In this program, our attempts are focused on understanding the contribution of Tfh cells and related subsets in driving the B cell output and antibody response in dengue. Dengue is an interesting model to understand the T-cell determinants of humoral immunity development. We are exploring the biology of Tfh-cell and related T-cell subsets in various outcomes of the dengue virus infection. Our current report from the patient cohort provides an insight into the cellular basis of antibody responses to dengue virus. We provide the first evidence of a peripheral T helper subset that showed the phenotypic resemblance to Tfh cells, and also the traits of help to B cells. We have developed a highly efficient antigen- specific T-B co-culture assay for measuring the T-dependent B cell responses, which work with as low as ~90 Ag-specific T cells (Cell Report Methods, 2022). We found that the novel Th-subset can provide help to B cells, has the potential of driving plasmablasts differentiation and induce the IgG production, as efficiently as the bonafide Tfh subset. We further observed that the novel Th-subset can induce productive cross-talk with both the naïve and memory B cells. We are now progressing to understand the transcriptomics landscape and the clonotype diversity of this newly identified CD4 + T cell subset at the single cell level, using 10X genomics platform. We also intend to develop a transgenic mouse model to further explore the origin of this novel subset.
Immunological memory to SARS-CoV-2 infection and vaccination
Understanding the T-cell immunity to SARS-CoV-2 infection and vaccination is important to develop effective pan-coronavirus vaccines. We reported within a few months of the pandemic that the Indian population seems to have high magnitude of the cross-reactive T cells that existed before COVID pandemic and may be generated due to exposure to common cold viruses. The high level of cross-reactive CD4+ T cells prior to SARS-CoV-2 infection perhaps explains the less severe disease and lower rates of hospitalization in India (Frontiers Immunology, 2021). We are now conducting detailed investigations on the T cells in individuals who recovered from COVID-19 (Asymptomatic and mild) and received vaccination.
We recently provided the first evidence of the immunological effectiveness of the inactivated whole-virion COVID-19 vaccine COVAXIN®. We found that the vaccine induces robust immune memory to SARS-CoV-2 that was comparable with that following SARS-CoV- 2 infection in the levels of antibodies, memory B cells and memory T cells, up to 6 months post-vaccination. and variants of concern that persist for at least 6 months after vaccination. Although the SARS-CoV-2 variants may substantially impact the vaccine-induced antibodies, the T-cell response were highly preserved. Interestingly, the vaccine is not capable of inducing CD8 T cells and they are substantially impacted by the variants. We also found that the vaccine generated Tfh cells that are endowed with B cell help potential, similar to the Tfh cells induced after natural infection. This study provides important knowledge for evidenced-based policymaking on the future application of COVAXIN ® (Nature Microbiology, 2022). We are now extending these studies to understand the biology of Tfh cells in cases of sustained immunological memory.
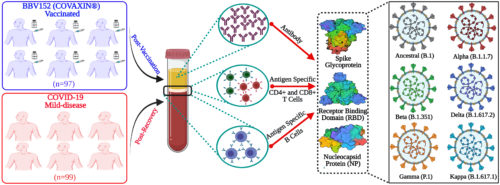
The study determined the immunological effectiveness and immune memory responses at least up to 6 months after immunization with indigenous COVID-19 vaccine “Covaxin”, and compared it with the recovery after natural infection. (Vikkurthi, R et.al., 2022. Nature Microbiology 7: 974-985).
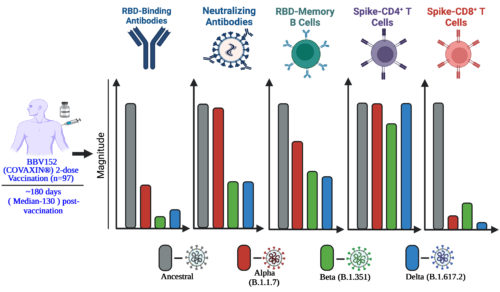
The study reported that COVID-19 vaccine “Covaxin” induced a robust immune memory against spike and nucleoprotein that was comparable with that following SARS-CoV-2 infection in the levels of antibodies, memory B cells and memory T cells, up to 6 months post vaccination. It also highlighted that the SARS-CoV-2 variants will impact the antibodies generated by vaccine, however, the T cells are not affected and may respond robustly against the variants. (Vikkurthi, R et.al., 2022. Nature Microbiology 7: 974-985).
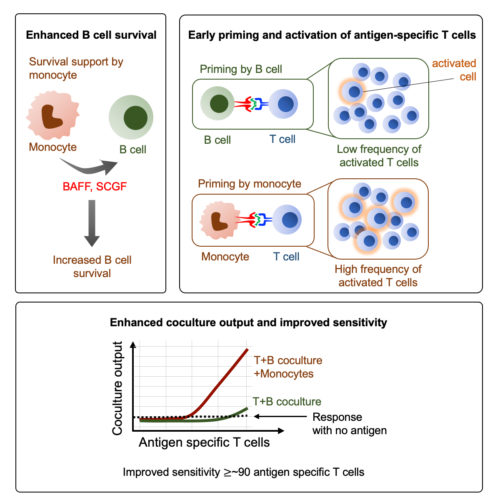
An Efficient immunoassay for the humoral immunity function of T cells.
There are several limitations in studying the human immune system. Particularly, the sample volume used to be a major limitation for performing multiple cellular analyses at the antigen-specific levels. Therefore, we continue to attempt on developing the immunological tools that can help us to examine the biology of antigen-specific T cells. In the similar efforts, we have recently described a highly sensitive and efficient T-cell assay that can measure the B cell help function of memory CD4 + T cells, with as few as ∼90 T cells. We have also provided mechanistic details of this enhanced method, which will be helpful in the widespread use of this assay in various vaccine and infection models including COVID-19 vaccines, Mycobacterium tuberculosis, Dengue etc. (Cell Reports Methods, 2022, Indian Patent). Utilizing this assay, we are now dissecting the crucial determinants of the productive cross-talk between T cells and B cells. We believe that the efforts will open up new avenues of research in the area of T-cell and B-cell collaboration.
Human Immune Monitoring and T-cell Immunoassay Platform
While the laboratory’s research has focused on the exciting questions summarized above, we have made contributions outside of the core research efforts of the lab. We have established a “Human Immune Monitoring and T-cell Immunoassay” platform, with applications for the advanced immunological human trials of vaccines and biotherapeutics. The platform includes a variety of assays for measuring antigen-specific T cells and B cells in human blood – quantitation of virus-specific T cells, T-cell phenotype, T-cell functions, quantitation of polyfunctional T cells, measurement of anti-viral T cells, T-cell ability in developing humoral immunity, quantitation of virus-specific memory B cells (IgA, IgM and IgG + B cells) etc. This is a significant contribution to vaccine research in the country. During the Covid-19 pandemic, the platform was instrumental in generating scientific evidence for the T-cell immunity to Covid-19 vaccines and supporting the policy making for vaccine implementation. When the vaccine efficacy studies were not feasible, this platform was instrumental in conducting the immuno-bridging phase 3 clinical trial under industry-academia partnership for testing intranasal COVID- 19 vaccine “iNCOVACC” in generating the systemic and mucosal cell-mediated immunity (CTRI/2022/02/039992). The platform was also used for the interchangeability trial of Japanese encephalitis vaccines. Moreover, the tools are now available for evaluating the dengue vaccine candidates for their ability in inducing the long term and good-quality humoral immunity.
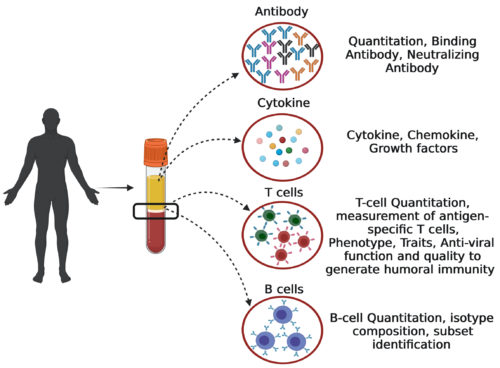
Human Immune Monitoring and T-cell Immunoassay Platform.
This platform includes the advanced human immunology techniques for human trials of
vaccines, biotherapeutics and the antivirals.
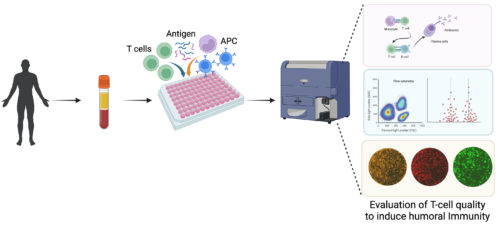
The immunoassay for the selection of promising vaccine candidates by testing their
ability to induce potent T cells and humoral immunity.
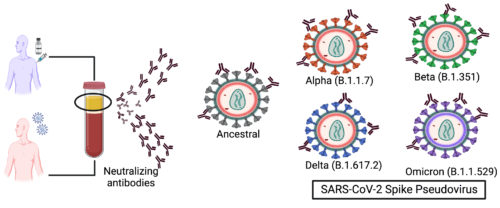
The SARS-CoV-2 Pseudovirus setup.
The SARS-CoV-2-Spike expressing Pseudoviruses. It includes both the lentivirus based and the VSV-backbone based pseudo viruses for measuring the virus neutralizing antibodies in infection and vaccination in BSL-2 settings.
Network







Funding

nbm

SERB

NII

HIPC

DBT-NERBPMC

DBT